
HL Paper 1
Which combination of properties is correct?

Markscheme
A
Examiners report
Which group of ions and molecules has delocalized electrons in all the species?
A. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{, }}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{\text{CO}}{{\text{O}}^ - }\) and \({{\text{O}}_{\text{3}}}\)
B. \({\text{NO}}_{\text{3}}^ - {\text{, NO}}_{\text{2}}^ - \) and \({\text{C}}{{\text{O}}_{\text{2}}}\)
C. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{, CO}}_{\text{3}}^{{\text{2}} - }\) and graphite
D. \({{\text{C}}_{\text{6}}}{{\text{H}}_{\text{6}}}{\text{, CO}}_{\text{3}}^{{\text{2}} - }\) and \({{\text{C}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
Markscheme
C
Examiners report
Which species contain dative covalent bonds?
I. CO
II. \({\text{N}}{{\text{H}}_{\text{3}}}\)
III. \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\)
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
B
Examiners report
Which sequence has the molecules in order of increasing nitrogen-nitrogen bond length?
A. \({{\text{N}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}}\)
B. \({{\text{N}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}}\)
C. \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}\)
D. \({{\text{N}}_{\text{2}}}{{\text{H}}_{\text{2}}} < {{\text{N}}_{\text{2}}}{{\text{H}}_{\text{4}}} < {{\text{N}}_{\text{2}}}\)
Markscheme
B
Examiners report
Which substance has the following properties?
• Low melting point
• Very soluble in water
• Does not conduct electricity when molten
A. Glucose, \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}{{\text{O}}_{\text{6}}}\)
B. Silicon dioxide, \({\text{Si}}{{\text{O}}_{\text{2}}}\)
C. Sodium chloride, NaCl
D. Tetrachloromethane, \({\text{CC}}{{\text{l}}_{\text{4}}}\)
Markscheme
A
Examiners report
Which metal has the strongest metallic bonding?
A. Na
B. Mg
C. Al
D. Ca
Markscheme
C
Examiners report
Which compounds have an ionic lattice structure in the solid state?
I. Silicon dioxide
II. Sodium fluoride
III. Ammonium nitrate
A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Which diagrams can be used to represent the Lewis (electron dot) structure of boron trifluoride?

A. I and II only
B. I and III only
C. II and III only
D. I, II and III
Markscheme
C
Examiners report
Well over 50% of the candidates gave the correct answer but a significant number (31%) gave answer A thus rejecting representation III.
What is the difference between the strength and the length of the carbon-oxygen bond in butanal and in butan-1-ol?
A. The bond in butanal is stronger and longer than in butan-1-ol.
B. The bond in butanal is weaker and shorter than in butan-1-ol.
C. The bond in butanal is weaker and longer than in butan-1-ol.
D. The bond in butanal is stronger and shorter than in butan-1-ol.
Markscheme
D
Examiners report
What is the formula of calcium nitride?
A. \({\text{C}}{{\text{a}}_{\text{3}}}{{\text{N}}_{\text{2}}}\)
B. \({\text{C}}{{\text{a}}_{\text{2}}}{{\text{N}}_{\text{3}}}\)
C. \({\text{Ca(N}}{{\text{O}}_{\text{2}}}{{\text{)}}_{\text{2}}}\)
D. \({\text{Ca(N}}{{\text{O}}_{\text{3}}}{{\text{)}}_{\text{2}}}\)
Markscheme
A
Examiners report
Which molecule has an octahedral shape?
A. SF6
B. PCl5
C. XeF4
D. BF3
Markscheme
A
Examiners report
The Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) is given below.
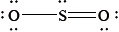
What is the shape of the \({\text{S}}{{\text{O}}_{\text{2}}}\) molecule?
A. Bent (V-shaped)
B. Linear
C. T-shaped
D. Triangular planar
Markscheme
A
Examiners report
There were two G2 comments on this question. One respondent stated that D. should be trigonal planar instead of triangular planar. Both terms are widely used in fact, though of course the correct answer is A. bent or V-shaped. Another respondent stated that it would have been better to represent the Lewis structure of \({\text{S}}{{\text{O}}_{\text{2}}}\) with valence expansion. It is true that \({\text{S}}{{\text{O}}_{\text{2}}}\) could be represented as an alternate Lewis structure. However, the question did not state what the best Lewis structure representation of \({\text{S}}{{\text{O}}_{\text{2}}}\) was and hence was not basing the representation at any distinction centred on formal charge differences versus expanded octets. Candidates simply had to look at the three negative charge centres present which equates to a triangular planar electron-domain geometry and hence a bent molecular geometry as the final shape giving A as the correct answer.
Which correctly lists butane \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propanone \({\text{(}}{M_{\text{r}}} = {\text{58)}}\), propan-1-ol \({\text{(}}{M_{\text{r}}} = {\text{60)}}\) and propan-2-ol
\({\text{(}}{M_{\text{r}}} = {\text{60)}}\) in order of increasing boiling point?
A. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}}\)
C. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}\)
D. \({{\text{C}}_{\text{4}}}{{\text{H}}_{{\text{10}}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(OH)C}}{{\text{H}}_{\text{3}}}\)
Markscheme
A
Examiners report
Most candidates were able to dismiss B and C but getting the correct answer required them to transfer their knowledge of steric hindrance (or alkane branching) to a different situation. 54% gave the correct answer whilst 30% gave D.
What is the correct order if the compounds are arranged in order of increasing boiling point?
A. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
B. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}}\)
C. \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{4}}}\)
D. \({\text{C}}{{\text{H}}_{\text{4}}} < {\text{Si}}{{\text{H}}_{\text{4}}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{Cl}} < {\text{C}}{{\text{H}}_{\text{3}}}{\text{OH}}\)
Markscheme
D
Examiners report
Zinc metal contains metallic bonding. Which is the best description of a metallic bond?
A. The electrostatic attraction between a pair of electrons and positively charged nuclei.
B. The electrostatic attraction between oppositely charged ions.
C. The electrostatic attraction between a lattice of positive ions and delocalized electrons.
D. The bond formed when one atom provides both electrons in a shared pair.
Markscheme
C
Examiners report
What is the bond angle in the \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) ion?
A. 104°
B. 107°
C. 109°
D. 120°
Markscheme
B
Examiners report
Which molecule contains a dative covalent (coordinate) bond?
A. HCN
B. \({{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}\)
C. \({\text{C}}{{\text{O}}_{\text{2}}}\)
D. CO
Markscheme
D
Examiners report
One respondent suggested that this question should be deleted as the question is based on an incorrect interpretation. However, in the teachers note corresponding to AS 4.2.2 examples such as CO, \({\text{N}}{{\text{H}}_{{\text{4 + }}}}\) and \({{\text{H}}_{\text{3}}}{{\text{O}}^ + }\) are clearly mentioned.
How many electrons form the carbon–oxygen bond in methanal, HCHO?
A. 2
B. 4
C. 8
D. 12
Markscheme
B
Examiners report
Four identical sealed containers are prepared each containing \({\text{10 c}}{{\text{m}}^{\text{3}}}\) of an organic compound and at the temperature shown below. Which container will have the highest vapour pressure?

Markscheme
D
Examiners report
In this question, candidates were asked to identify the container with the highest vapour pressure from a list of two substances and related temperatures. One of the substances was \({\text{C}}{{\text{H}}_{\text{3}}}{\text{OC}}{{\text{H}}_{\text{3}}}\), methoxymethane. One respondent stated that ethers are off-syllabus. This is an important point that has been addressed before in a number of subject reports. It is true to say that in the current programme candidates are not required to either identify ethers as a formal functional group or to name them, applying IUPAC rules. However, it should be noted that ethers are referred to in the TN corresponding to AS 4.3.2 in relation to the difference in boiling point between ethanol and methoxymethane, with regard to hydrogen bonding considerations. Hence, it is perfectly valid for methoxymethane to be cited in this particular question on vapour pressure.
How many atoms is each carbon directly bonded to in its allotropes?

Markscheme
B
Examiners report
A solid has a melting point of 1582 °C and does not dissolve in water. It does not conduct electricity in the molten state. What type of structure does the solid have?
A. Ionic
B. Metallic
C. Giant molecular
D. Simple molecular
Markscheme
C
Examiners report
Which combination describes the bonding and structure in benzoic acid, C6H5COOH?
Markscheme
B